Background: CPX-351, has been approved for the treatment of patients diagnosed with Acute Myeloid Leukemia (AML) arising from a previous myelodysplastic syndrome (s-AML) or secondary to chemotherapy (t-AML) as per former WHO 2016 classification, following results from the phase III trial (Lancet et al, JCO2018). Long term results from the trial confirmed that the benefit from CPX-351 over conventional 3+7 induction was maintained both in patients proceeding or not to allogeneic stem cell transplantation consolidation (HSCT). However, the information on the optimal duration of treatment with CPX-351 or the best timing for HSCT consolidation are still incomplete. Furthermore, there is also scarce data on the efficacy of CPX-351 among NPM1 or FLT3-ITD mutated AML or in patient with low risk AML according to European Leukemia Net (ELN) classification, as those subgroups are rare among t-AML and s-AML.
Aims: The aim of this study is to analyze the outcome of CPX-351 treatment in a large cohort of patient who received commercially available treatment in Italy since the approval of the drug, in order to identify the optimal duration of treatment, the best timing for HSCT and to evaluate the efficacy among more rare secondary AML subtypes.
Methods: 513 elderly (median age 65.6 years, range 19-79) s-AML or t-AML patients who received CPX-351 treatment in 38 Italian Centers since January 2019 were retrospectively included in this study. All patients received CPX-351 as per Italian Drug Authority (AIFA) approval, allowing up to two induction cycles and up to two consolidation. Diagnostic workup was performed as per internal standard in all patients. Eligible patients proceeded to HSCT consolidation as per internal standard of each Center. 108 (21.1%) and 405 (78.9%) patients were diagnosed with t-AML or s-AML, respectively. NPM1 mutation were found in 31 patients (6%), FLT3-ITD mutation was present in 24 patients (4.6%). ELN 2017 score was favorable, intermediate or high in 27 (5.2%), 177 (34.5%) and 309 (60.3%) patients, respectively. Most patients had relevant comorbidities (84%), mainly cardiovascular disease (43%), type II diabetes (39%).
After induction 1, 297/513 patients (58%) achieved a complete remission (CR). 72 patients failing to achieve CR received induction 2. After induction 2, CR was achieved in 340/513 patients (66.3%). CR rate was significantly higher among NPM1 mutated patients (p <0.05) and among ELN 2017 favorable risk patients if compared to intermediate or high risk (p<0.05), whereas was not affected by FLT3-ITD mutations. Among responding patients, 118 (34.7%), 137 (40.3%) and 85 (25%) received none, one or two consolidation cycles, respectively. HSCT consolidation in first CR was performed in 166/340 responding patients (48.8%). 30 and 60-days mortality were 5.2 and 8.2%, respectively.
After a median follow-up of 23.66 months (CI 95% 23.11 - 26.01), median OS was 16.23 months (CI 95% 13.6 - 18.9).
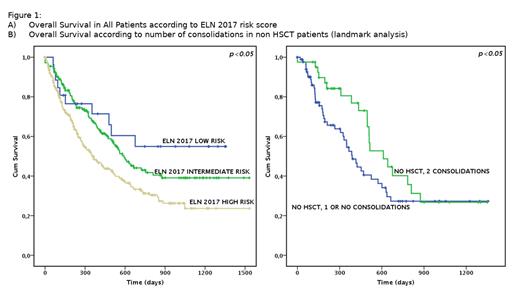
OS was significantly influenced by NPM1 mutational status (p<0.05) and ELN 2017 risk score (p<0.05, Fig. 1A). Of note, NPM1 mutated patients and ELN 2017 low-risk patients showed a very good outcome (Median OS 24.6 months for NPM1 mutated patients and not reached in ELN 2017 favorable risk patients).
In a landmark analysis including patients alive and in CR at day 90, HSCT was the strongest predictor of longer survival (Median OS not reached and 16.3 months for patients receiving or not HSCT, respectively, p<0.05). In the same landmark model, completion of all allowed CPX-351 treatment was beneficial only in patients not proceeding to HSCT (median OS 20.36 and 12.2 months in patients receiving 2 or less CPX-351 consolidation without HSCT, respectively, p<0.05, Fig. 1B), whereas in patients receiving HSCT consolidation further CPX-351 treatment after cycle 1 did not improve results (Median OS not reached, 35 and 28.4 months in HSCT patients receiving 0, 1 or 2 CPX-351 consolidations, respectively, p=n.s.)
Conclusions: Our large cohort confirms the efficacy of CPX-351 treatment and suggests that CPX-351 may be beneficial also in NPM1 mutated and ELN 2017 favorable risk patients. In eligible patients, HSCT should probably be performed as soon as a CR is achieved, whereas patients not proceeding to HSCT may still have a long survival if two consolidations are administered. In a future perspective, maintenance strategies may further improve the results.
Disclosures
Martelli:Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Laboratoires Delbert: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria. Lussana:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Speakers Bureau; Clinigen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees; Amgen: Speakers Bureau. Breccia:Incyte: Honoraria; AbbVie: Honoraria; Novartis: Honoraria; AOP: Honoraria; Pfizer: Honoraria; BMS: Honoraria. Bocchia:Novartis: Honoraria; Incyte: Honoraria; BMS: Honoraria. Galimberti:Abbvie, Janssen, Novartis, Roche, Jazz, Astra Zeneca, Pfizer, Incyte: Speakers Bureau. Palmieri:Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Vetro:Jazz Pharmaceuticals: Honoraria; BMS: Honoraria; ABBVIE: Honoraria. Zappasodi:Amgen, Pfizer, Abbvie, Astellas: Honoraria. Borlenghi:AbbVie, BMS: Consultancy; Amgen, Incyte: Other: travel grants. Cerrano:Insight Novartis Servier Abbvie Janssen Jazz Astellas Italfarmaco: Honoraria. Papayannidis:Abbvie, Astellas, Servier, Menarini/Stemline, BMS, Pfizer, Amgen, Janssen, Incyte, Novartis: Honoraria; Pfizer, Astellas, Janssen, GSK, Blueprint, Jazz Pharmaceuticals, Abbvie, Novartis, Delbert Laboratoires: Membership on an entity's Board of Directors or advisory committees. Alati:AbbVie: Honoraria; Jazz: Honoraria. Fracchiolla:Abbvie, Jazz, Pfizer, Amgen: Other: travel grants; Abbvie, Jazz, Pfizer, Amgen: Speakers Bureau. Ferrara:ABBVIE: Honoraria. Venditti:Medac: Consultancy; Janssen: Consultancy, Honoraria, Other: travel support ; AbbVie: Consultancy, Honoraria, Other: travel support ; Jazz: Consultancy, Honoraria, Other: travel support ; Amgen: Consultancy, Honoraria, Other: travel support ; Pfizer: Consultancy, Honoraria, Other: travel support , Speakers Bureau; Novartis: Consultancy, Honoraria, Other: travel support . Pagano:Janseen: Honoraria; Pfizer: Honoraria; Gilead: Honoraria; Jazz: Honoraria; Novartis: Honoraria; Menarini: Honoraria; Moderna: Honoraria; AstraZeneca: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal